Mental Health & Autoimmune Disease
A report publish in JAMA Psychiatry estimates that autoimmune disease increases risk of receiving a subsequent mood disorder diagnosis by 45%. Although, in some cases, this may be the psychological result of the limited circumstances that many suffering from autoimmune disease are faced with, it may prominently be a physiological response due to the underlying inflammation that accompanies autoimmune disease. The mood disorder, then, is a symptom of autoimmune disease, or a secondary condition, rather than an independent disease or condition.
What this means is that many of us may develop mental illness, such as anxiety, depression or OCD, as a consequence of our autoimmune disorders. In some cases the mental illness can become a more complicated issue than the autoimmune disease. For example, celiac disease is particularly well linked to the development of schizophrenia, if left untreated. Hyperthyroidism, hypothyroidism, rheumatoid arthritis, and polymyalgia rheumatica are prevalent in bipolar disorder. There are also positive associations between dementia and Addison’s disease, multiple sclerosis, psoriasis and systemic lupus erythematous.
So what is the connection between autoimmune disease, inflammation and mental health?
Well, there are several considerations here. Let’s break it down:
Th1 and Th2 Imbalances
First, it is important to understand that autoimmune disease occurs when there is overactivity of the immune system, most prominently of T-helper cells. T-helper cells (Th) are a lymphocytes (types of white blood cells) that recognize foreign pathogens. In autoimmune disease, these are the cells that flag your body’s normal tissue as foreign pathogens and create inflammatory cytokines to mount an attack… oopsies!
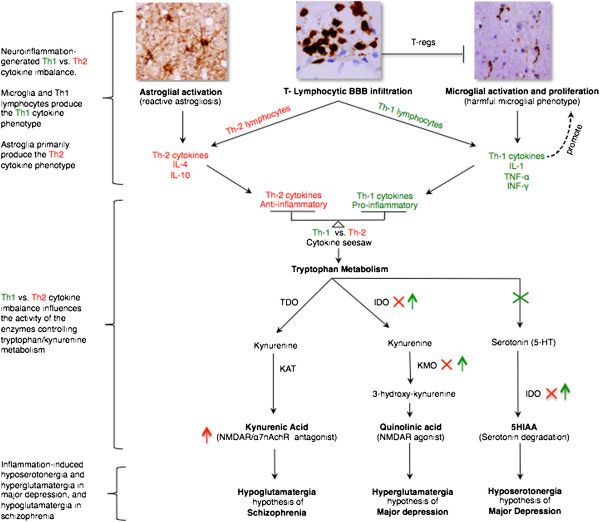
There are two kinds of Th cells that are meant to maintain a balance with one another. Th1 cells are part of the “cell-mediated” immune system, which is in charge of dealing with infections by viruses and certain bacteria. Th1 cells tend to be pro-inflammatory and are involved in the development of organ-specific autoimmune diseases like celiac disease, hashimoto’s thyroiditis, crohn’s disease and psoriasis.
Th2 cells are involved in what’s called the “humoral” immune system, which deals with bacteria, toxins and allergens. They are in charge of stimulating the production of antibodies in response to pathogens found in the blood or other body fluids. Th2 cells tend not to be inflammatory and are involved in systemic autoimmune disease like lupus and scleroderma and ulcerative colitis.
In an optimized immune system, both groups of T-cells work together to maintain balance. In autoimmune disease, however, we tend to see a dominance to either the Th1 or Th2 pathway. When Th1 cells of the immune system are overactive, they can suppress the activity of the Th2 cells and vice versa. This imbalance can provoke attack on healthy tissue and worsen autoimmune symptoms.
Similar imbalances have been observed in those with psychiatric disorders. This draws a connection between autoimmune disease and these immunological abnormalities leading to inflammation and contributing to psychiatric disorders. For example, in major depressive disorder, we see a Th1 dominance, leading to the proliferation of inflammatory cytokines (particularly IL-1 and TNF-alpha). These inflammatory cytokines have the ability to block the conversion of tryptophan to serotonin and increase serotonin degradation leading to hyposerotonergia. Hyposerotonergia is a primary hypothesis of major depressive disorder characterized by a deficiency of serotonin production and/or utilization.
On the other side of the coin, Th2 dominance may be associated with schizophrenia due to the creation of IL-4 and IL-10 anti-inflammatory cytokines and their mechanisms that funnel tryptophan metabolism into kyneurenic acid, which leads to hypoglutamatergia. Hypoglutamatergia is a major hypothesis behind schizophrenia, characterized by a deficiency of glutamate production and/or utilization.
SOURCE: Najjar, Souhel, et al. “Neuroinflammation and Psychiatric Illness.” Journal of Neuroinflammation, vol. 10, no. 1, 2013, doi:10.1186/1742-2094-10-43.
SO, if you have an autoimmune disease that has created an imbalance between Th1 and Th2 immunity, this imbalance may contribute to the development of these two mental illnesses. Ironically, it’s a bummer.
Leaky Gut and the Blood-Brain-Barrier
The second big connection being made is the association between autoimmune disease, leaky gut, inflammation and a degradation of the blood-brain-barrier (BBB). For all autoimmune diseases in which it’s been tested, a leaky gut is present. It is now functionally considered a prerequisite to the development of autoimmune disease. Unfortunately, the presence of a leaky gut may allow harmful bacteria, toxins and undigested food particles through to the bloodstream where they may be circulated to the brain.
There are many contributors to a leaky gut but perhaps the most prominent is gluten. Gluten-exposure stimulates a protein called zonulin, which triggers an opening of the tight junctions (little structures that hold the cells of your gut lining together), essentially creating a “hole” in the gut lining through which these undesirable substances can enter the bloodstream.
If all of these things happen and you now have inflammatory substances floating through the bloodstream, when they reach the brain, the blood-brain-barrier (BBB), should be able to keep them out. However, this zonulin protein also modulates the BBB, so if a gluten protein survives the trip, it could trigger what is now being identified colloquially as a “leaky brain”.
As it turns out, this “leaky brain” is being observed in several mental health disorders. An elevated “cerebral spinal fluid (CSF): serum albumin ratio” in patients with major depressive disorder and schizophrenia suggests increased BBB permeability. In one study, involving 63 pychiatric subjects with depression, bipolar disorder and schizophrenia and 4,100 controls, CSF abnormalities indicative of BBB-damage were detected in 41% of psychiatric subjects.
Inflammation, Oxidative Stress & Mitochondrial Dysfunction
Due to our overactive and imbalanced immune systems, active autoimmune diseases are correlated with elevated levels of inflammation and the production of pro-inflammatory cytokines. In major depressive disorder we see elevated TNF-alpha, IL-6 and sIL-2R during depressive episodes. In bipolar, we see elevated IL:6 during depressive states, IL-4 during euthymic state and IL-2, IL-4 and IL6 during manic states. In schizophrenia, we see elevated IFN-y, TNF-a, IL-12 AND sL-2R.
The production of these pro inflammatory cytokines may contribute further to the promotion of reactive oxygen species (ROS), which in turn accelerates lipid peroxidation, damaging membrane phospholipids and their membrane-bound neurotransmitter receptors and depleting antioxidants. Increased ROS products can also enhance the production of inflammatory cytokines, creating more oxidative injury and a consistent pathological feedback loop observed in some psychiatric disorders.
These pro-inflammatory cytokines, such as TNF-alpha, can also reduce mitochondrial density and impair mitochondrial oxidative metabolism. Mitochondrial dysfunction, also common in autoimmune disease likely due to the shared proliferation of these inflammatory cytokines, may further contribute to increased oxidative stress in conditions like major depressive disorder, bipolar disorder and schizophrenia.
Why are you telling me this, Megan!?!!?
Ok, so I know that this information might feel a bit overwhelming and, ironically, might get you down. The good news is, if we can implement an anti-inflammatory protocol through interventions that address gut health, immune balancing, oxidative stress and other root causes, we can reduce the inflammatory cycle that creates the mental illness in the first place, all while likely improving the condition of your autoimmune disease as well.
A few things we can do to get started?
Consider a gut-healing protocol
I would recommend looking at a paleo, if not full AIP diet, to address leaky gut. As mentioned above, gluten is the primary offender for creating a leaky gut. However, all grains and legumes contain lectins, digestive enzyme inhibitors and phytic acid, which can contribute to dysbiosis and the development of a leaky gut.
But aside from just eliminating things from you diet, I would look at the things we can INCLUDE to heal the gut, like bone broth, organ meats and plenty of wild-caught seafood.
I highly recommend working with an informed practitioner to ensure you are getting everything you need, and avoiding everything you don’t need (at least for a time!)
Implement some immune-balancing strategies
Identify if you are Th1 or Th2 dominant and avoid compounds that stimulate the dominant side of your immune system. For example, if you are Th2 dominant, caffeine may exacerbate the issue while ginseng might help to balance it. So if you’re looking for an energy boost, you would go with the ginseng instead of the coffee and the opposite would be true if you are Th1 dominant.
Do your research and consult a health care practitioner for help with this.
Reduce oxidative stress
Avoid environmental stimulants for oxidative stress like cigarette smoke and chemical exposure from common toxic household items like tile cleaner, makeup, deodorant, etc. Avoid consuming oxidized fats, fried or burnt foods. Again, more importantly, make sure you are getting your antioxidants. Aim to consume as much as you can from your food, but you may also consider supplementation. NAC is particularly well studied for its ability to reduce oxidative stress in both autoimmune disease and mental health disorders. NAC is N-Acetyl Cysteine a free form amino acid that facilitates the production of glutathione, your body's master antioxidant involved in fighting oxidative stress, maintaining a healthy immune system, repairing tissues and supporting overall health. Since supplemental glutathione is extremely difficult to absorb, assimilate and use, we have seen greater improvements in serum glutathione levels with supplementation of N-Acetyl Cysteine.
These are just a few of the things we can do. Some things are out of our control but there is a LOT that is within our control. It’s up to us to take that control.
Please feel free to leave any questions or comments below. I know there is a lot to unpack here. I’m here for you.
Kisses & Kombucha,
Meg
SOURCES
Adams, Case. “Are Mental Disorders the Result of Neuroinflammation?” GreenMedInfo, 3 July 2015, https://www.greenmedinfo.com/blog/are-mental-disorders-result-neuroinflammation.
Al-Diwani, Adam A.J., et al. “Psychosis: an Autoimmune Disease.” Immunology, vol. 152, 5 July 2017, pp. 388–401., doi:10.111/imm.12795. https://www.ncbi.nlm.nih.gov/pubmed/28704576
Dalmazi, Giulia Di, et al. “Reactive Oxygen Species in Organ-Specific Autoimmunity.” Autoimmunity Highlights, vol. 7, no. 1, 2016, doi:10.1007/s13317-016-0083-0. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4974204/
Najjar, Souhel, et al. “Neuroinflammation and Psychiatric Illness.” Journal of Neuroinflammation, vol. 10, no. 1, 2013, doi:10.1186/1742-2094-10-43. https://jamanetwork.com/journals/jamapsychiatry/fullarticle/1696348
Neumann, H. “Control of Glial Immune Function by Neurons.” Glia, U.S. National Library of Medicine, Nov. 2001, https://www.ncbi.nlm.nih.gov/pubmed/11596127.
Skerrett, Patrick J. “Infection, Autoimmune Disease Linked to Depression.” Harvard Health Blog, 17 June 2013, https://www.health.harvard.edu/blog/infection-autoimmune-disease-linked-to-depression-201306176397.
Trescott, Mickey. “How Do You Balance Th1 and Th2 in Autoimmune Disease?” Autoimmune Wellness, 7 Sept. 2015, https://www.autoimmunewellness.com/how-do-you-balance-th1-and-th2-in-autoimmune-disease/.
Wotton, Clare J, and Michael J Goldacre. “Associations between Specific Autoimmune Diseases and Subsequent Dementia: Retrospective Record-Linkage Cohort Study, UK.” Journal of Epidemiology and Community Health, vol. 71, no. 6, 2017, pp. 576–583., doi:10.1136/jech-2016-207809. https://jech.bmj.com/content/71/6/576.long